By utilizing genome-edited mice, I aim to identify the causes of hereditary diseases in canines and develop "genetic surgery" treatments.
*Information related to faculty members/students and graduate schools at Gifu University here are all that of the time of interviewing.

Using advanced gene analysis and genome editing technologies, I am working on elucidating the sex-determining factors in mice and applying this technology to study hereditary diseases in canines. Through identifying the mutations that cause hereditary diseases, I aim to establish "gene therapy through genome editing" in the future.
The true factor determining the sex of mice has been revealed.

I entered the veterinary course at Gifu University because I wanted to treat animal diseases. Even within the same breed of dog, there are differences in size, shape, and color, and the diseases they develop also vary by breed. There are many unknowns about animal diseases, and I wanted to uncover these mysteries and understand the wonders of animals. As an undergraduate, I found genetics "complicated" and challenging, but now I am conducting research in this field.
In recent years, to learn research methods for handling non-model organisms -neither mice nor humans - I have participated in research using induced pluripotent stem (iPS) cells to study the naked mole-rat, which is long-lived and cancer-resistant, at the genetic level. To further understand the research, I studied the sex-determining genes in mice using advanced genome editing.
In 1990, it was discovered that the sex of mammals is determined by the presence or absence of the Sex-determining region Y (Sry). For the past 30 years, it has been believed that the Sry gene is composed of a single exon*1. However, our research team, using the latest analysis and genome editing technologies, analyzed mice and discovered an unknown transcript in the Sry gene of mice. This unknown transcript*2 includes a previously unidentified second exon (hidden exon), and we revealed that the Sry-T, which records this, is the true sex-determining factor in mice (a transcript leading to the second exon of Sry) (Figure 1). This achievement was recognized, and we received the "Young Scientist Award" from the Minister of Education, Culture, Sports, Science and Technology in the field of science and technology.
With our research having elucidated the complete picture of the Sry gene, which holds the key to sex determination, it is expected that this will advance our understanding of the mechanisms of sex determination in mammals, the evolution of sex-determining genes, and human sex differentiation disorders.

It has been revealed that the newly identified Sry-T, rather than the previously known Sry-S, functions as the true sex-determining factor. This was confirmed by the fact that XY mice lacking Sry-T (on the left in the photo) became female, XX mice expressing Sry-T (center) changed sex to male, and XX mice expressing Sry-S remained female (right).
We aim to identify the genes responsible for disease onset in canines and develop genetic surgery treatments.
We are currently focusing on research to replicate hereditary diseases in dogs using genome-edited mice. About 400 hereditary diseases have been reported in dogs, but the genetic mutations which cause about 70% of these diseases have not been identified. I would like to uncover as many of these mutations as possible. However, even if we decode the genome sequence and estimate the causative mutations, it is technically and ethically impossible to genetically modify dogs to prove that these mutations are indeed the cause. Therefore, we are working on creating mice that develop the same diseases as dogs through genome editing and analyze these mice to identify the causes of hereditary diseases.
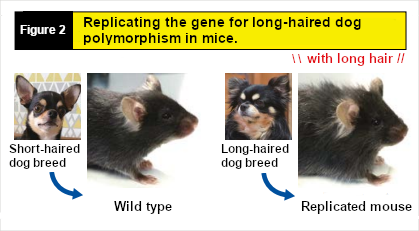
For example, by changing just one base in the genome sequence of a mouse to mimic the gene for long hair in dogs, the mouse will grow long hair like the dog (Figure 2). The same approach can be applied to size. Therefore, we plan to create genome-edited mice with genes for miniaturization to elucidate the mechanisms of obesity commonly seen in small dogs. Miniaturization in dogs can be considered analogous to "short stature" in humans. Studying diseases in canines can also lead to understanding human diseases. So far, by analyzing the genomes of specific dog breeds and replicating them in mice, we have identified about five causative gene mutations. In the future, we aim to optimize genome editing treatments in mice and establish "genetic surgery" for dog cases through genome editing.
This series of studies, titled 'Creation of Trans-Species Biology Realized by Genome-Edited Mice,' has been selected for this year's 'Fusion Oriented Research for Disruptive Science and Technology (FOREST)' of the Japan Science and Technology Agency. Recently, we have formed a partnership with Associate Professor Junji Moribe from the School of Social System Management and Associate Professor Tatsunori Masatani from the Laboratory of Zoonotic Diseases, Faculty of Applied Biological Sciences, to clarify the mechanisms of viral infection at the molecular level using mice with replicated genome sequences of wild animals. We will continue to conduct research on hereditary diseases and morphological characteristics of various animals, and we hope that the knowledge gained from animals can be applied to human medicine in the future. To find the next "big" thing, we will devote ourselves to the tasks at hand. I encourage students to cherish their excitement and curiosity and to be eager to learn. There is no better time to learn what you want to know than now.
 Developing surgical models for practical training
Developing surgical models for practical trainingSince 2020, the Veterinary Surgery Laboratory has been working on developing surgical models for dogs. These models allow each student to repeatedly use them by replacing inexpensive disposable parts, enabling practical training that closely resembles actual surgery without using live animals. This initiative aims to promote new veterinary education and contribute to animal welfare. This project has received significant support through crowdfunding.





